How to Use Melting Points to Identify the Melting Point of the Compounds Using a Melting Point Apparatus
Essay by MuHaMmAd Bin JaAFaR • November 11, 2018 • Lab Report • 1,159 Words (5 Pages) • 1,849 Views
Essay Preview: How to Use Melting Points to Identify the Melting Point of the Compounds Using a Melting Point Apparatus
ABSTRACT
The purpose of this experiment was to understand how to use melting points to identify the melting point of the compounds using a melting point apparatus, to understand what occurs at the molecular level when a substance melts, to understand the primary purpose of melting point data and to demonstrate the technique for obtaining the melting point of an organic substance. To compare the melting point of a compound, we must know what the expected melting point value for each of the compound first. Two compound was selected which is EDTA and gallic acid.
INTRODUCTION
We tend to think of solids as those materials that are solid at room temperature. However, all materials have melting points of some sort. Gases become solids at extremely low temperatures, and liquids will also become solid if the temperature is low enough. Solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. However, while liquids are fluid, solids are not. The particles of most solids are packed tightly together in an orderly arrangement. The motion of individual atoms, ions, or molecules in a solid is restricted to vibrational motion about a fixed point. Solids are almost completely incompressible and are the densest of the three states of matter.
As a solid is heated, its particles vibrate more rapidly as the solid absorbs kinetic energy. Eventually, the organization of the particles within the solid structure begins to break down and the solid starts to melt. The melting point is the temperature at which a solid changes into a liquid. At its melting point, the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid. As with boiling points, the melting point of a solid is dependent on the strength of those attractive forces. Sodium chloride (NaCl) is an ionic compound that consists of a multitude of strong ionic bonds. Sodium chloride melts at 801°C. Ice (solid H20) is a molecular compound whose molecules are held together by hydrogen bonds. Though hydrogen bonds are the strongest of the intermolecular forces, the strength of hydrogen bonds is much less than that of ionic bonds. The melting point of ice is 0°C.
The melting point of a solid is the same as the freezing point of the liquid. At that temperature, the solid and liquid states of the substance are in equilibrium. However, freezing points are rarely measured in practice because they are more difficult to determine. One reason for this is that solidification may not occur at the correct temperature due to the phenomenon of super cooling. Super cooling occurs when a liquid is cooled below its freezing point does not solidify. Determination of the temperature at which the solid and liquid phases of a substance are in equilibrium is tedious and time consuming; it is also quite difficult with a small amount of sample. Thus, in practice, most melting points are determined as capillary melting points, which can be done quickly with a small amount of sample in a capillary tube. A capillary melting point is defined as the temperature range over which a small amount of solid in a thin walled capillary tube first visibly softens (first drop of liquid) and then completely liquefies.
OBJECTIVE
- To determine the melting point of the mixture of EDTA and Gallic acid.
- To observe the physical and chemical changes during the heating
CHEMICALS
- Ethylenediaminetetraacetic acid (EDTA)
- Gallic Acid
APPARATUS
- Melting point apparatus
- Electronic weighing machine
- Capillary tubes
PROCEDURE[pic 1]
RESULT
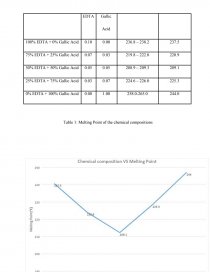
Chemical Composition | Mass (g) | Standard Melting point range(°C) | Melting Point (°C) | |
EDTA | Gallic Acid | |||
100% EDTA + 0% Gallic Acid | 0.10 | 0.00 | 236.8 – 238.2 | 237.5 |
75% EDTA + 25% Gallic Acid | 0.07 | 0.03 | 219.8 – 222.0 | 220.9 |
50% EDTA + 50% Gallic Acid | 0.05 | 0.05 | 208.9 – 209.3 | 209.1 |
25% EDTA + 75% Gallic Acid | 0.03 | 0.07 | 224.6 – 226.0 | 225.3 |
0% EDTA + 100% Gallic Acid | 0.00 | 1.00 | 258.0-265.0 | 244.0 |
Table 1: Melting Point of the chemical compositions
[pic 2]
Table 2: Graph Chemical Composition VS Melting Point
...
...